 Tristan Driscoll
Tristan Driscoll
Abstract
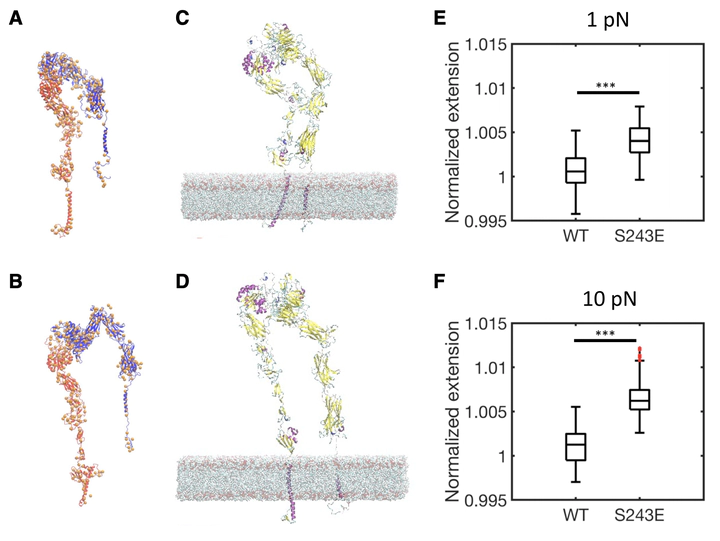
Conversion of integrins from low to high affinity states, termed activation, is important in biological processes, including immunity, hemostasis, angiogenesis, and embryonic development. Integrin activation is regulated by large-scale conformational transitions from closed, low affinity states to open, high affinity states. Although it has been suggested that substrate stiffness shifts the conformational equilibrium of integrin and governs its unbinding, here, we address the role of integrin conformational activation in cellular mechanosensing. Comparison of wild-type versus activating mutants of integrin αVβ3 show that activating mutants shift cell spreading, focal adhesion kinase activation, traction stress, and force on talin toward high stiffness values at lower stiffness. Although all activated integrin mutants showed equivalent binding affinity for soluble ligands, the β3 S243E mutant showed the strongest shift in mechanical responses. To understand this behavior, we used coarse-grained computational models derived from molecular level information. The models predicted that wild-type integrin αVβ3 displaces under force and that activating mutations shift the required force toward lower values, with S243E showing the strongest effect. Cellular stiffness sensing thus correlates with computed effects of force on integrin conformation. Together, these data identify a role for force-induced integrin conformational deformation in cellular mechanosensing.